Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
[ad_1]
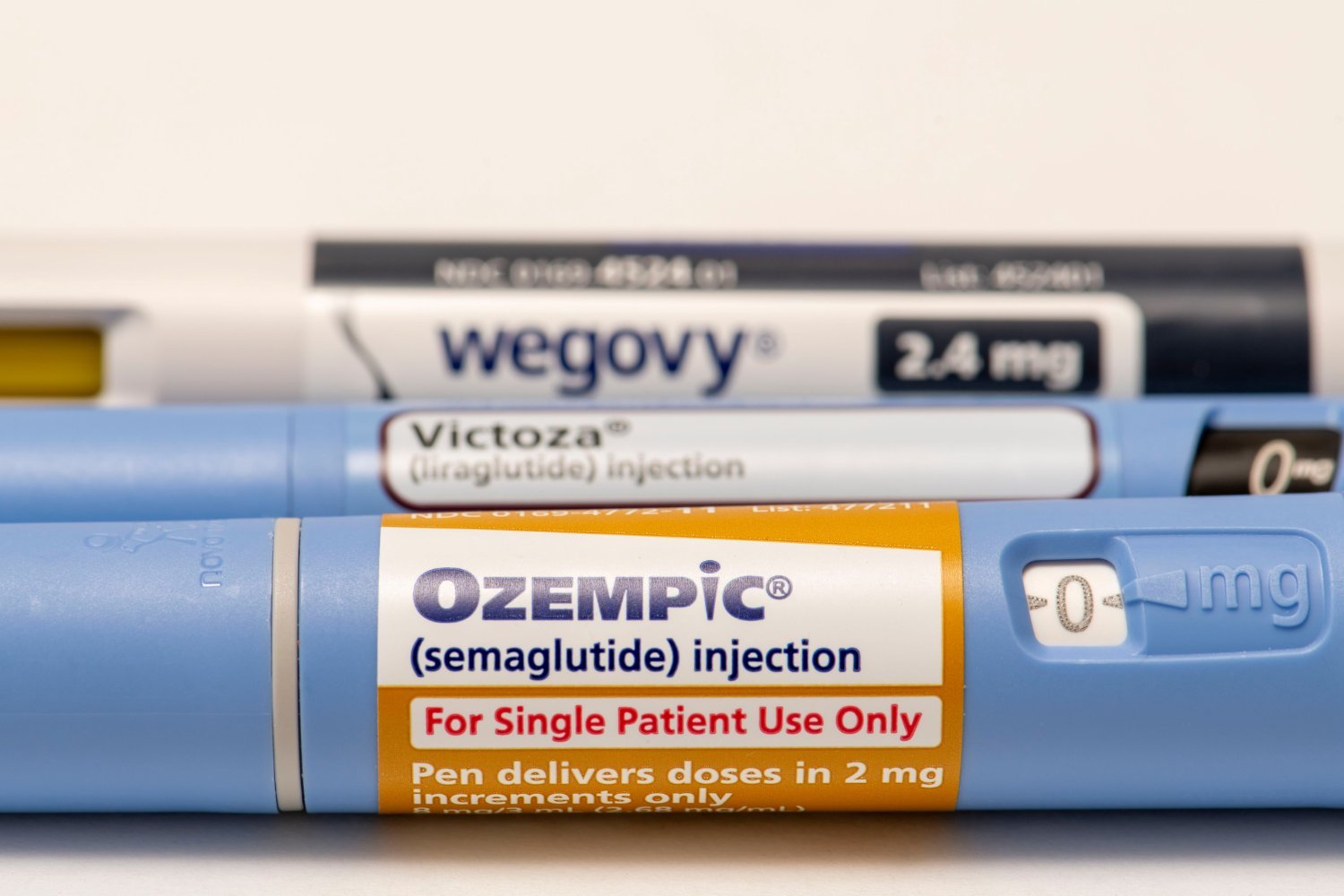
The active ingredient of popular drugs is designed to add an active ingredient in Ozempic and Wegovy, to add to the list of growing medical usage. In a large-scale clinical test, the semaglutide, liver disease was found to be effective in treating a severe and relatively dissemination form.
Researchers in the United States and Britain have led the Phase III Court, funded by the Novo Nordisk. Compared to the placebo, the semaglutide has significantly improved the results of people with steatohepatitis related to metabolic dysfunction (MASH). Findings will lead to the path of semaglutide and similar medications to have front treatment for this chronic situation.
MASH is the heaviest form of steepotic liver disease associated with metabolic dysfunction (MASLD). There are both chapting In the liver, most of the extreme rainfall or permanent itching or permanent itch or liver can cause cirroz damage. These conditions were previously recognized in previous non-alcoholic steatohepatitis (NASH) and non-alcoholic oil.
MASLD is the most common form of the most common liver disease Most people are advancing for mashed, but the situation still affects American adults to 6.5%. The cirroz caused by Nash can raise the risk of other heavy complications, including Hepatosellu carcinoma (the most common form of major liver cancer) and open liver failure.
MASLD / PASH can cause several factors, including a person’s genetic genetic, but there are both obesity and diabetes great contributor. There are 90% of people with people overweight and 90% of people with severe obesity; There are only two-thirds of the two-thirds of the type of diabetes. Given this close connection, scientists have hoped that there are semaglutide and similar medications that imitate Hormone GLP-1 (already approved to treat) Obesity and diabetes) MASLD / MASH can be an effective treatment.
Novo Nordiskin Phase III Court, named Öriyah, 800 patients mixed with 800 patients. Volunteers were randomly instructed to buy a placebo in the highest dose of seemaglutide (up to 2.4 milligrams, the highest dose of obesity) or 72 weeks. Both groups also received lifestyle advice.
The end of the study, two-thirds of the people in the semaglutide, two-thirds of steatohepatitis (liver accompanied liver) doubled the same percentage of those who live the same in Plasebo. People in the drug also improved the liver fibrosis (building wound tissue), more improved in placebo and lost more weight (average 10%). People experienced similar adverse effects in the semaglutide as it seems mostly in the past trials with gastrointestinal symptoms such as nausea, diarrhea and vomiting.
“I have been working with GLP-1 for sixteen years, and these results are a growing problem in the world. statement from the university. “Although these results are not treated cautiously, analysis semaglutide can be an effective tool to treat these advanced liver diseases.”
The findings of the courts released on Wednesday in the New England Medical Magazine are almost certain to ensure the approval of medication to treat puree from medicine and drug leadership. Expected approval will only represent the latest copies for a recent condition that has not yet been treated. A year ago, FDA confirmed The first such medication, rezdafra from Madrigal Pharmacy.
The resffdifra is slightly different in terms of how to treat puree. A different receptor is targeting that preventing oil from the liver without weight. However, it is most likely positive, because more treatment options for choosing doctors and patients, especially when people do not respond well to one or the other, they must give more treatment options.
Other GLP-1 drugs (including) Medications that imitate hormones associated with other weight) tested for mashed. Thus, if things continue to go well, these drugs can soon revolutionize the treatment of obesity for obesity.
[ad_2]
Source link