Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
[ad_1]
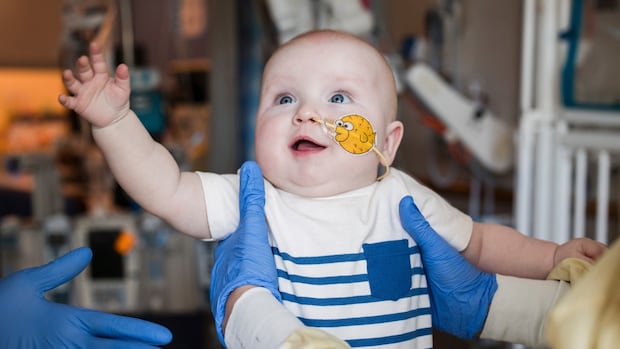
A born with a rare and dangerous genetic disease, according to doctors who treat it, grow and develop after the experimental gene editing treatment.
Researchers said that a new study in a new study said that the work in a new study, which killed half of the infants with a small but critical mistake, he said. Although it may be a while before, it belongs to others for others, doctors, technology for one day, because it is so rare, it can lead to a day behind genetic medicine.
“This is the first step for the use of Gene regulatory therapy to treat various rare genetic disorders, because it is not yet clear medical treatment,” Dr. Kiran Musunuru, Pennsylvania Gene Edit University, Education Specialist on Thursday New England Medical Magazine.
Clifton Heights’s KJ Muldoon, Penn., One of the most rare diseases is one of 350 million people in the world. Some experts were diagnosed shortly after birth with a serious CPS1 deficiency to influence one of a million babies.
In those babies do not have an enzyme needed to help the ammonia remove from the body, so they are building and toxic in their blood. Liver transplant is an option for some.

Know the bets of KJ, parents are worried about Kyle and Nicole Muldoon, both 34, they can lose it.
“We knew, we know, pulling all the options, by asking any questions for something that was invasive or not previously made before,” said Nicole.
“We prayed, we talked to people, collect information, and as a result, we are the way we went to go,” Her husband added.
During the six months, the team in Philadelphia and Penn Medical Children’s Hospital created a therapy designed to correct KJ’s wrong gene with his partners. Gene regulation, which won the moral morality in 2020, used a Gene regulation vehicle that cut the adjustment vehicle in 2020. Like the first crisp rapprochements, doctors used a technique known as “letter” – a technique known as the correct type. Recognized as “base editing”, it reduces the risk of expected genetic changes.
The team’s therapy created so quickly “Very excited”, Senthil Bhoopalan Senthil Bhoopalan in Senthil Bhoopalan, who did not participate in the study. “It really carries the pace and criteria sets for such approaches.”
In February, KJ received the first IV infusion by Gene regulatory therapy through small oil drops called Lipid nanoparticles taken by liver cells.
The UK drug regulator has allowed the first gene therapy for the world’s first gene therapy for two blood disorders – the first gene therapy for the orak cell and thalassemia. CASGEVY is a licensed first medicine using a crisp crisper, which won the Nobel Prize in 2020.
The room reminded the author of the call, “He slept with the whole thing,” he said.
After the Takla UP doses in March and April, KJ was well healed better than diseases such as colds, which could aggravate the body and aggravate the body. A child nine and a half a month of a month also takes little medicine.
Given the weak forecast before, “We also see the smallest stage that we met at any time – there is a big point for us – it’s like a little wave or rolling.”

Again, researchers have been a few months for a few months. They will have to watch him for years.
“It’s still a lot to understand what this medicine can do for KJ,” Ahrens-Nicklas said. “But every day, he shows us that he grew up.”
Researchers hope that they learn from KJ to help other disease patients.
Development is extremely expensive, generally targeting more common reasons for simple financial reasons: more patients can pay more potentially more potential to pay and earn more earnings. For example, the first crisp therapy approved by the US food and drug setting is treated with a painful blood disorder that affects the sickle cell disease, millions around the world.
Musunuru, the work of the team does not need to be banned the work of the United States, creating a special treatment by US National Health Institutes. He said that the US $ 800,000 was “not far,” the United States was worth $ 800,000 for medium liver transplant and related care.
“I would take place in the economy of scale and waiting for the economy of scale, because we have better improvement and even better the time.
Scientists will not have to redesign all preliminary work every time you create a special therapy, so this research sets “stage” for other rare conditions.
CARLOS Moraes, a neurologist Carlos Moraes, who did not associate with the research, opened the door to progress more like this investigation.
“Once someone comes with such progress, it will not take time,” he said, “I have agreements for other teams to apply and move forward.” Then it will move as a block because we are very ready, because the whole area will move as a block. “
[ad_2]
Source link